预防,无疑是任何污染控制策略(CCS)的核心,它涉及了多个方面,包括实施严格的卫生程序、对原料和包装的严密控制,以及精心的设施设计。然而,除了这些预防措施外,生产流程中能够尽早识别潜在的污染风险也极为关键。
环境监测在此过程中扮演了至关重要的角色。通过在设施内部进行实时监测,任何污染迹象都能被迅速捕捉,从而触发相应的纠正措施。对于用户而言,污染问题的及时发现意味着能够最大限度地减少产品损失。特别地,在药品生产领域,EU Annex 1为污染控制策略制定了具体的标准和要求。这些标准旨在帮助生产企业识别、评估并控制潜在的污染威胁,以确保药品的质量和安全性。
在这个背景下,MicronView凭借其在空气监测领域的深厚知识和丰富经验,为我们带来了BAMS(浮游菌实时监测系统)。高级应用专家Christine Troutman将与我们分享如何利用BAMS来优化污染控制策略,以满足EU Annex 1的标准,进而减少生产过程中的损失。
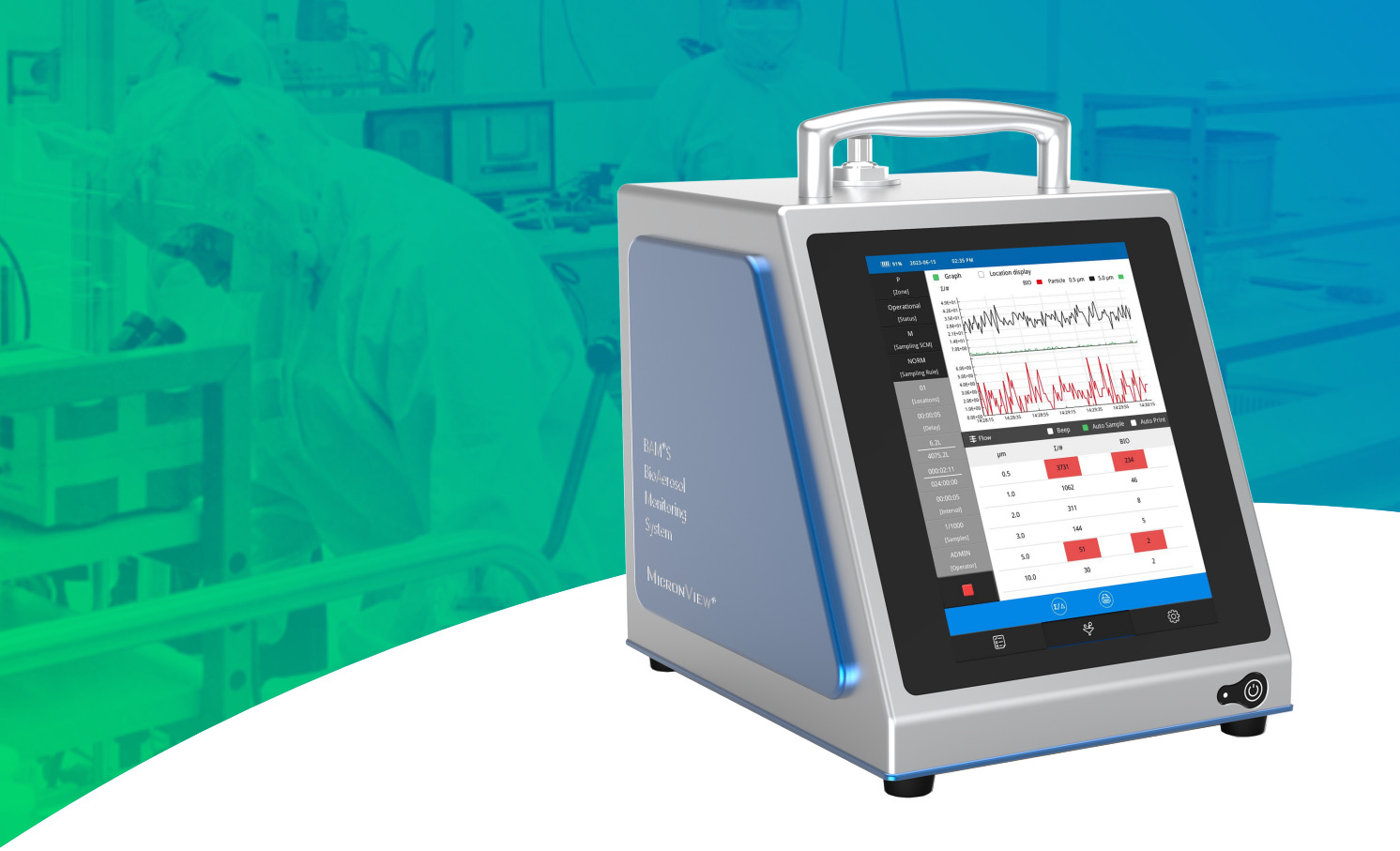
Q: How does the BioAerosol Monitoring System (BAMS) work?
Christine:BAMS is a type of Biofluorescent Particle Counter (BFPC). BFPCs determine the biologic status and size of particles in air samples using fluorescence and light scattering. Particles are drawn into the instrument and scanned by a laser beam, which causes the light to scatter around the particle. This scattered light pattern is read by the instrument, and the size of the particle is determined.
Simultaneously, if the particles are viable, they will have metabolites (such as NADPH and Riboflavin) that are excited by the laser, causing them to emit fluorescence. This fluorescent signal is then detected and analyzed by the instrument. By analyzing the fluorescence and scattered light, the instrument can differentiate between biological and non-biological particles, providing real-time monitoring of microbial contamination in environments like pharmaceutical facilities and cleanrooms.
提问:浮游菌实时监测系统 (BAMS) 是如何工作的?
Christine:BAMS是一种生物荧光粒子计数器(BFPC),专门用于监测空气中微生物污染的设备。它结合了荧光技术和光散射原理,以确定空气样本中粒子的生物状态和大小。
工作原理:
粒子采样与激光扫描:BAMS首先吸入空气样本,然后利用激光束对样本中的粒子进行扫描。当激光束照射到粒子时,会在粒子周围产生散射光。
光散射分析:BAMS的仪器读取这些散射光信号,并通过分析这些信号来确定粒子的大小。
荧光检测:如果粒子是具有活性的微生物,它们会含有代谢物(如NADPH和核黄素)。当这些代谢物被激光激发时,会发出荧光。BAMS的仪器能够检测并分析这种荧光信号。
应用与功能:
BAMS通过分析荧光和散射光信号,能够区分生物粒子(如微生物)和非生物粒子。这使得它在制药设施、洁净室等需要高度控制微生物污染的环境中具有极高的应用价值。通过对这些环境中的微生物污染进行实时监测,BAMS有助于确保产品质量。
Q: What advantages does it have over conventional air sampling instruments?
Christine:BAMS offers several advantages over conventional growth-based contamination control strategies. Firstly, BAMS provides instantaneous results compared to the days required for traditional culture methods to produce visible colonies. This rapid detection allows for immediate corrective actions, minimizing the risk of product loss or contamination. This saves not only a significant amount of time but also production or product loss costs. Additionally, BAMS offers higher sensitivity, capable of detecting low levels of microbial contamination that may be missed by culture-based methods, as well as the ability to detect viable but not culturable (VNBC) organisms. This sensitivity is crucial in environments where even small microbial counts can pose a significant risk, such as in pharmaceutical manufacturing.
Furthermore, BAMS automates the counting and sizing of particles, reducing the potential for human error and increasing the reproducibility of results. BAMS also does not require any reagents or sample preparation, thus saving cost, time and increasing sustainability. Overall, BAMS provide a faster, more sensitive, and automated approach to contamination control, enhancing the efficiency and reliability of microbial detection in critical environments.
提问:与传统的空气采样仪器相比,它有哪些优势?
Christine:与传统的基于生长的污染控制策略相比,BAMS确实具有多项显著优势。
首先,BAMS提供了即时结果,与传统培养方法需要数天才能产生可见菌落相比,这是一个巨大的优势。这种快速检测的能力使得用户能够立即采取纠正措施,最大限度地降低产品损失或污染的风险。这不仅为用户节省了宝贵的时间,还减少了生产或产品损失成本,提高了整体运营效率。
其次,BAMS具有更高的灵敏度。它能够检测基于培养的方法可能会漏检的低水平微生物污染,并且还能检测可存活但不可培养(VNBC)的微生物。这种灵敏度在制药等环境中尤为重要,因为这些环境中即使是很小的微生物数量也可能带来严重的风险。通过BAMS的高灵敏度检测,用户可以更加准确地了解生产环境中的微生物污染情况,从而能够更早地采取更加有效的控制措施。
此外,BAMS还能自动计数和确定粒子大小,减少了人为失误的可能性,提高了结果的可重复性。这降低了对操作人员的依赖,使得监测结果更加准确可靠。同时,BAMS不需要任何试剂或样品制备,从而节省了成本和时间,提高了监测过程的可持续性。
综上所述,BAMS提供了一种更快、更灵敏、更自动化的污染控制方法。它提高了关键环境中微生物检测的效率和可靠性,为用户提供了更加有效的污染控制手段。在制药、医疗、食品等需要高度控制微生物污染的行业中,BAMS的应用将会越来越广泛。
Q: Do the results need confirmation? How is that done?
Christine: Once BAMS is validated at the customer site, which involves a process of comparing BAMS to the current air sampling procedure, results obtained by the device are considered accurate. According to Annex 1, any microbial contamination should be identified if possible, so secondary air sampling with a traditional growth-based method can be conducted if an alert or action level is reached on BAMS. This allows the user to confirm the presence of contaminating microbes and attempt to identify the contaminating species. However, the majority of microorganisms present in the environment may not be culturable, so BAMS may detect the presence of organisms that the traditional air sampling device cannot.
提问:结果需要确认吗?如何确认?
Christine:一旦在客户现场对BAMS进行了验证,包括与当前的空气采样程序进行比较,并且验证结果确认BAMS的准确性和可靠性,那么该设备所提供的结果就可以被认为是准确的。
根据Annex 1的规定,任何微生物污染都应尽可能加以识别和控制。因此,如果BAMS在监测过程中达到警报或行动级别,表示可能存在微生物污染的风险,此时使用传统的生长法进行二次空气采样是一种常见且有效的方法。
传统的生长法可以帮助用户确认是否存在污染微生物,并尝试确定污染物的种类。然而,值得注意的是,环境中存在的大多数微生物可能无法在传统的培养条件下生长,因此它们可能会被传统的空气采样设备所遗漏。而BAMS正是为了弥补这一不足而设计的,它能够检测到那些可存活但不可培养(VNBC)的微生物,以及低水平的微生物污染。
通过结合BAMS和传统生长法的使用,用户可以更全面地了解环境中的微生物污染情况,并采取更加有效的控制措施。BAMS的即时性和高灵敏度使得用户可以迅速响应并降低污染风险,而传统的生长法则可以提供更加详细的微生物信息,帮助用户深入了解污染物的种类和特性。这种综合应用的方式将有助于提高微生物检测的准确性和可靠性,保障生产环境的安全和产品质量。
Q: Which regulatory requirements can the BAMS help comply with?
Christine: There are many regulatory guidelines that BAMS can help users comply with, including but not limited to EU GMP Annex 1, ICH Q9 – Quality Risk Management, FDA Guidance – Sterile Drug Products Produces by Aseptic Processing, USP <1116> Microbiological Control and Monitoring of Aseptic Processing Environments, and ISO 14698 – Cleanrooms and Associated Controlled Environments.
BAMS offers significant benefits for compliance with the new regulations outlined in Annex 1. It enables real-time environmental monitoring, providing immediate feedback on airborne microbial contamination levels, a new requirement of Annex 1. In Grade A cleanroom environments, BAMS can be used as a complementary environmental monitoring tool in the detection of viable particles where 0 CFU counts are permitted. In all other cleanroom environments, BAMS also provides particle sizing and counting capabilities, aiding in risk assessment by identifying different types and sizes of particles that may carry microorganisms.
This rapid detection of contamination allows for prompt corrective actions to prevent product contamination, aligning with the requirements outlined in Annex 1 and the associated Contamination Control Strategy (CCS). Furthermore, BAMS complements traditional monitoring methods by offering continuous, digital monitoring data for improved data integrity and traceability, essential for regulatory compliance and audit purposes.
提问:BAMS可以帮助遵守哪些监管要求?
Christine:BAMS确实在帮助用户遵守多个监管准则方面发挥了重要作用,这些准则包括但不限于EU GMP Annex 1、ICH Q9(质量风险管理)、FDA指南关于无菌加工生产的无菌药品、USP<1116>无菌工艺环境的微生物控制和监测,以及ISO 14698关于洁净室和相关受控环境的标准。
BAMS为遵守Annex 1中的新规定提供了显著优势。它可以进行实时环境监测,提供有关空气中微生物污染水平的即时反馈,这一功能是Annex 1中特别强调的新要求。在A级洁净室环境中,BAMS可以作为一个辅助环境监测工具,用于检测允许CFU计数为0的可存活粒子。而在所有其他洁净室环境中,BAMS还能够提供粒子大小测定和计数功能,通过识别可能携带微生物的不同类型和大小的颗粒,帮助用户进行风险评估。
这种快速检测污染的方法使得用户能够迅速采取纠正措施,防止产品污染,从而符合Annex 1和相关污染控制策略(CCS)的要求。与传统的培养方法相比,BAMS不仅能够提供更快的检测结果,还能够检测那些基于培养方法可能会漏检的低水平微生物污染以及可存活但不可培养(VNBC)的微生物。
此外,BAMS提供的连续数字监测数据提高了数据完整性和可追溯性,这对于满足监管合规性和审计目的至关重要。通过连续监测,用户可以实时了解所监测环境的微生物污染状况,并据此采取必要的控制措施。这种实时监测和连续数据记录的功能使得BAMS成为传统监测方法的有力补充。
Q: What maintenance and calibration are needed?
Christine: BAMS requires very minimal maintenance if operated correctly. The exterior of the unit can be cleaned with commonly used disinfectants in the cleanroom environment. A yearly calibration is required to ensure both fluorescence detection and particle sizing abilities are functioning accurately.
提问:需要进行哪些维护和校准?
Christine:当操作正确时,BAMS只需要极少的维护。这大大降低了用户的运营成本和时间投入。
设备外部清洁:
BAMS的外部可以使用洁净室环境中常用的消毒剂进行清洗。这有助于保持设备的外观整洁,并减少外部污染物对设备性能的影响。在进行清洁时,应确保所使用的消毒剂与BAMS的材质兼容,并按照厂家建议进行操作。
年度校准:
为了确保BAMS的荧光检测和颗粒大小测定能力始终保持在准确状态,每年需要进行一次校准。这一步骤至关重要,因为它能够确保BAMS提供的检测结果是可靠和准确的。校准应由专业的技术人员进行,他们将使用标准的校准方法和设备来验证BAMS的性能。
Q: How do facilities start integrating the BAMS into their CCS? What validations are needed?
Christine: Integrating BAMS into a new or existing Contamination Control Strategy (CCS) requires a thorough but straightforward validation process at the user site. This involves conducting a comparison study between the BAMS and existing air sampling technology to validate its functionality and establish alert and action levels. Additionally, a risk assessment study should be conducted to ensure that the BAMS is deployed in appropriate zones based on contamination risk levels.
提问:设施如何开始将 BAMS 集成到其中央监控系统中?需要进行哪些验证?
Christine:将BAMS集成到新的或现有的污染控制策略(CCS)中需要在用户现场进行全面而直接的验证过程。这包括对BAMS和现有空气采样技术进行比较研究,以验证其功能并确定警报和行动级别。此外,还应进行风险评估研究,以确保根据污染风险等级将BAMS部署在适当的区域。
将BAMS集成到新的或现有的污染控制策略(CCS)中确实需要在用户现场进行全面而直接的验证过程。以下是验证过程的关键步骤和考虑因素:
BAMS与现有空气采样技术的比较研究:
- 对BAMS和现有的空气采样技术(如传统的生长法、被动式沉降菌采样和主动式浮游菌采样器等)进行直接的比较研究。
- 验证BAMS在检测微生物污染方面的准确性、灵敏度和特异性。
功能验证:
- 验证BAMS的实时环境监测功能,确保其能够即时提供空气中微生物污染水平的反馈。
- 验证BAMS的粒子大小测定和计数功能,确保其能够识别可能携带微生物的不同类型和大小的颗粒。
- 检查BAMS的数据输出功能,确保其能够提供清晰、准确的监测数据和报告。
警报和行动级别的确定:
- 根据比较研究和用户的具体需求,确定BAMS的警报和行动级别。
- 这些级别应该基于微生物污染的风险水平、产品的重要性以及用户对风险的容忍度等因素进行设定。
风险评估研究:
- 进行风险评估研究,以确定BAMS应部署在哪些区域以最大程度地降低污染风险。
- 考虑不同区域的微生物污染风险等级、产品暴露程度以及人员活动等因素。
- 根据风险评估结果,制定BAMS的部署计划和监测策略。
通过上述验证过程,用户可以确保BAMS能够准确地集成到他们的污染控制策略中,并为其提供有效的微生物监测解决方案。
Q: There are several real-time bioaerosol monitors on the market; what advantages does the BAMS offer?
Christine: BAMS offers some key advantages over other Biofluorescent Particle Counters (BFPCs) on the market. First, BAMS is highly portable, weighing only 15lbs/7kgs and having a battery life of approximately 6 hours. The small size of BAMS (255H X 200W X 264D mm) makes it extremely easy to integrate into current cleanroom systems. The portability of BAMS also makes it easy to conduct root cause investigations. Furthermore, BAMS also has an advanced software algorithm that serves to reduce false positive biological counts by reducing interferent signals. This is highly beneficial in cleanroom environments where even low counts may require an investigation. Finally, BAMS has an 8” touchscreen that can be used with two layers of gloves, allowing users to view results in real time and edit sampling plans with the simple and streamlined user interface.
提问:市场上有几种实时生物气溶胶监测仪,BAMS 有哪些优势?
Christine:与市场上的其他生物荧光粒子计数器(BFPC)相比,BAMS确实具有一系列显著的优势,这些优势使其在洁净室环境中的应用更为便捷和高效。以下是BAMS的主要优势:
便携性与轻量设计:
- BAMS的重量仅为15lbs/7Kg,电池运行时长约6小时。
- 小巧的体积(255H X 200W X 264D mm)让BAMS非常容易集成到当前的洁净室系统中,无需对现有设施进行大规模改造。
- 便携性还使得BAMS在需要进行根源调查时能够迅速部署到指定区域,快速定位污染源。
先进的软件算法:
- BAMS配备了先进的软件算法,能够有效减少干扰信号,从而降低假阳性生物计数。在洁净室环境中,低计数的准确性尤为重要,因为即使少量的微生物污染也可能对产品质量产生重大影响。
实时反馈与易用性:
- 8英寸触摸屏设计允许用户在戴两层手套的情况下仍能轻松操作,这使得在洁净室环境下进行操作变得更加方便和舒适。
- 实时查看功能让用户能够即时了解监测结果,并根据需要进行快速响应。
- 简单流畅的用户界面使得编辑采样方案变得轻而易举,无需复杂的操作步骤。
高效性与灵活性:
- BAMS的便携性和实时反馈功能使得用户能够高效地进行微生物监测工作,及时发现并处理潜在问题。
- 灵活的采样方案编辑功能使得BAMS能够适应不同洁净室环境的监测需求,提供定制化的解决方案。
这些优势使得BAMS成为洁净室环境中不可或缺的监测工具之一。
Q: Can you share a user experience where the BAMS has been implemented and a clear ROI has resulted?
Christine: One significant benefit of using BAMS is during root cause investigations, which can lead to a clear return on investment (ROI). Unlike traditional growth-based sampling methods that take days to process and identify contamination, BAMS provides rapid results, facilitating quick discovery of the contamination source. This capability is particularly crucial as delays in identifying the cause could result in product loss. For instance, Pfizer conducted a case study in 2018 at a site in Puerto Rico following Hurricane Maria, aiming to return to Good Manufacturing Practice (GMP) conditions quickly and accurately. During this study, BAMS was used to scan HEPA filters and detect areas with increased biologic or particulate counts, indicating filter failure. This proactive approach to filter inspection helped Pfizer identify and replace at-risk filters promptly, minimizing delays and retests. The study demonstrated how BAMS can save time and resources, enhancing the process of returning to GMP conditions after an unplanned shutdown.
提问:您能否分享一下已实施 BAMS 并获得明确投资回报的用户体验?
Christine:使用BAMS在根源调查期间的一个显著优势是其能够迅速提供结果,从而带来明确的投资回报率(ROI)。与传统的基于生长的采样方法相比,BAMS能够显著减少等待时间,使污染源能够更快地被发现和处理。在洁净室环境中,任何微生物污染都可能对产品造成严重影响,甚至导致产品损失。因此,快速查明污染源对于保持生产效率和产品质量至关重要。
以辉瑞公司在波多黎各的一个生产基地为案例,该公司在飓风“玛丽亚”过后使用BAMS来快速恢复良好生产规范(GMP)条件。在这个案例中,BAMS被用于扫描HEPA过滤器,检测生物或粒子数增加的区域,从而迅速发现过滤器故障。通过积极主动的过滤器检测方法,辉瑞公司能够及时发现并更换存在风险的过滤器,避免了进一步的延误和重新检测。这项案例研究展示了BAMS在根源调查中的强大作用。通过快速提供准确的监测结果,BAMS帮助辉瑞公司节省了时间和资源,加强了在意外停产后恢复到GMP条件的过程。这种能力使得BAMS成为一种高效、可靠的监测工具,为用户带来了显著的投资回报率。

Christine Troutman是MicronView的高级应用科学家,对于生物荧光粒子计数器和其他环境监测设备的应用具有丰富的经验。她拥有科罗拉多州立大学微生物学和免疫学硕士学位,在加入MicronView 团队之前曾从事肿瘤诊断和疫苗开发工作。
